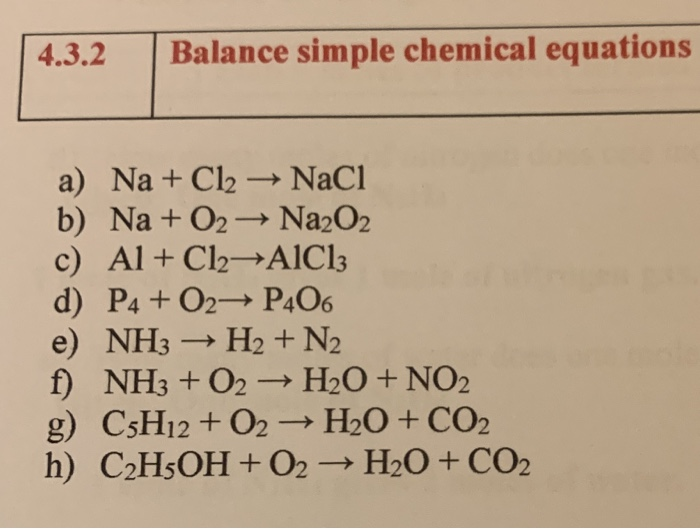
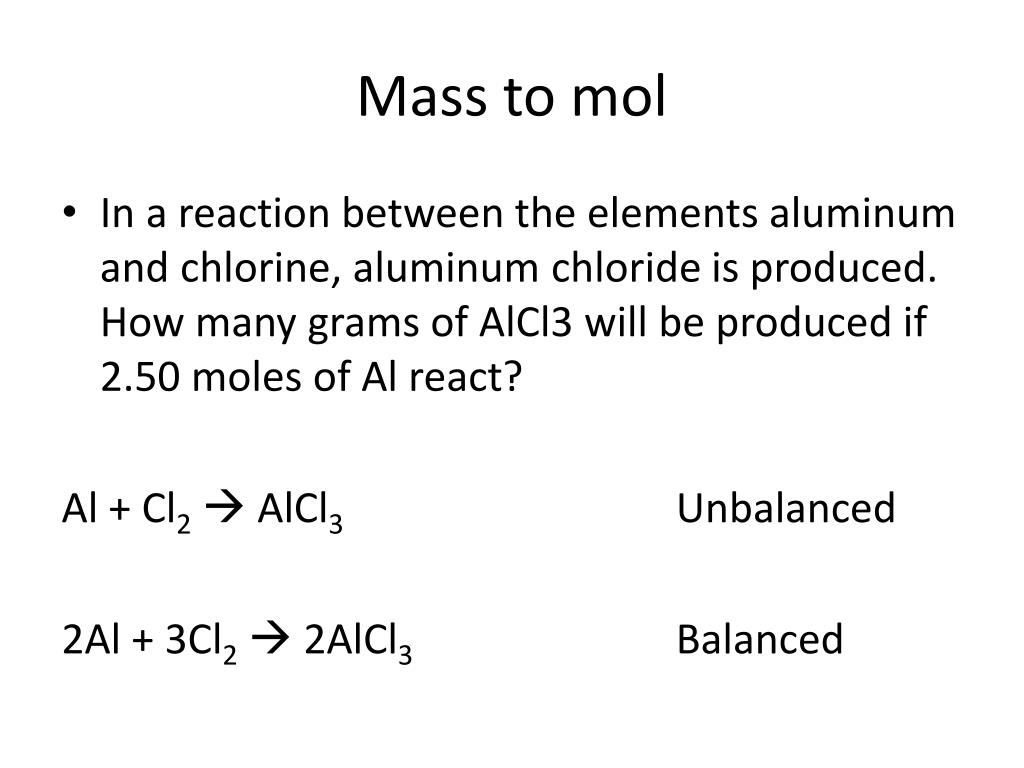
SOLVED: Be sure to answer all parts. The balanced equation for the reaction of aluminum metal and chlorine gas is: 2Al(s) + 3Cl2(g) â†' 2AlCl3(s) Assume that 0.48 g Al is mixed
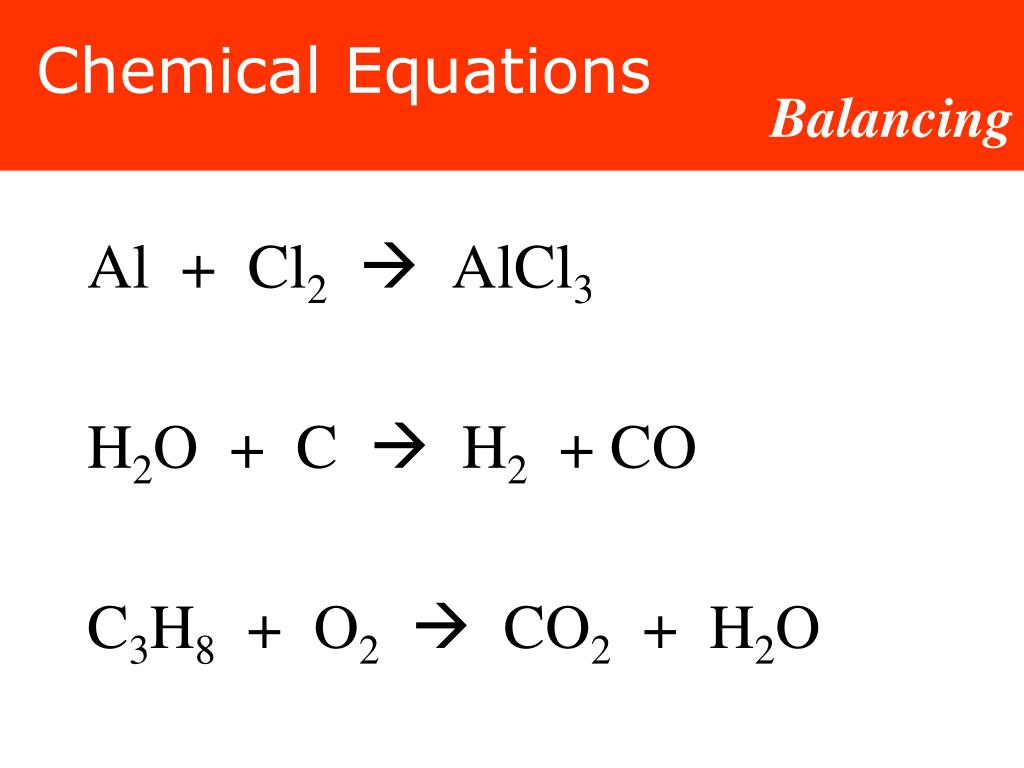
PPT - Like a recipe: Reactants Products 2H 2 (g) + O 2 (g) 2H 2 O(l) PowerPoint Presentation - ID:3558136

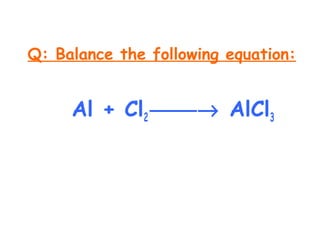
SOLVED: Write a balanced equation for the following reaction: Al(s) + Cl2(g) â†' AlCl3(aq) + Cl2(aq) a. 2 Al(s) + 3 Cl2(g) â†' 2 AlCl3(aq) + 6 Cl2(aq) b. Al(s) + 2
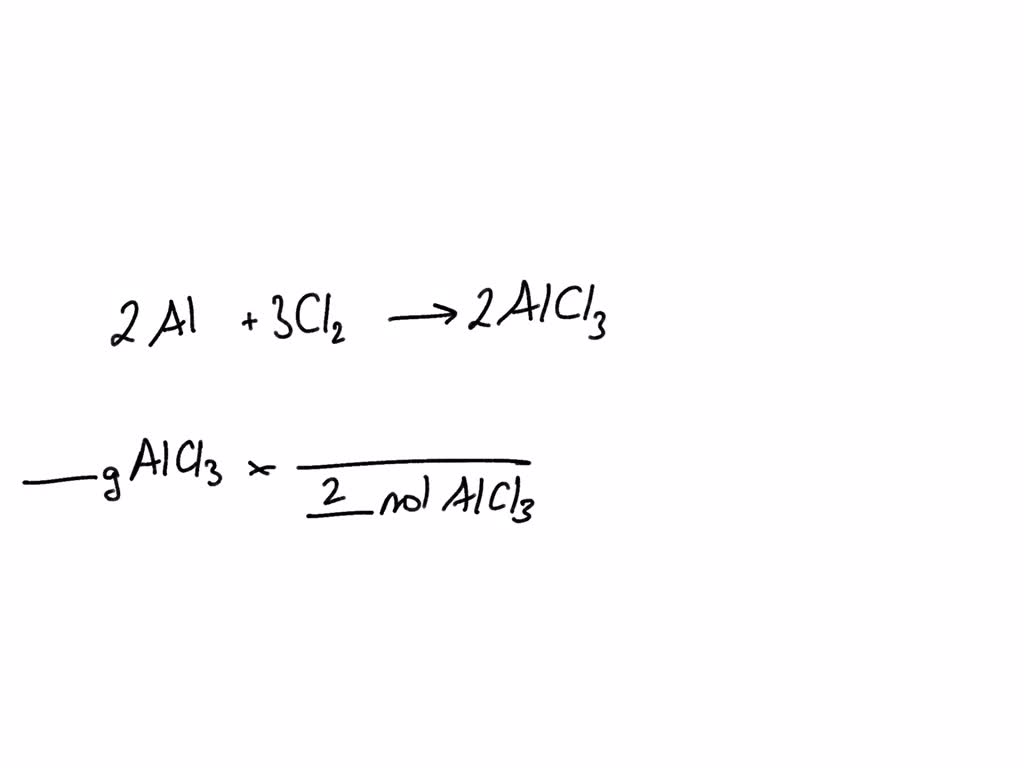
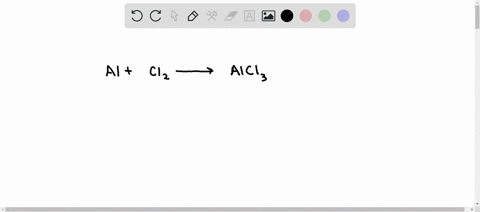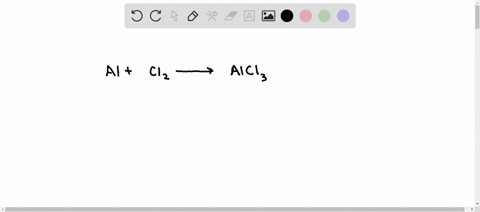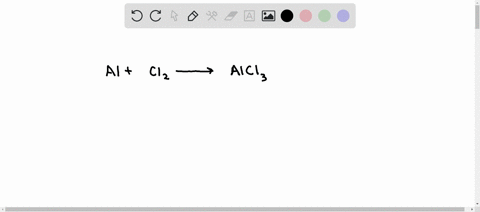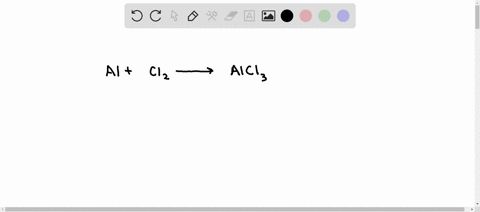
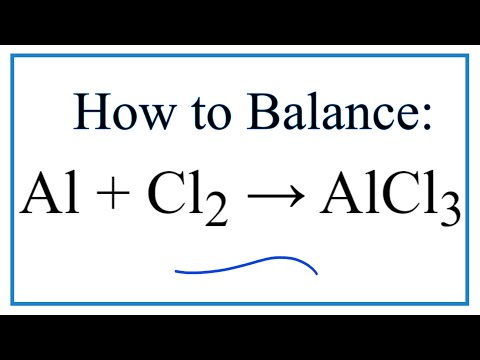
SOLVED: Aluminum reacts with chlorine gas to produce aluminum chloride according to the following equation. Al + Cl2 → AlCl3 Which of the following fractions can be used for the mole ratio